WuXi AppTec Named “Industry Mover” in S&P Global Sustainability Yearbook 2023
2023-02-16
WuXi AppTec Medical Device Testing Center is a specialized, integrated testing platform that brings extensive expertise and experience in the medical device field. We provide a broad spectrum of services that include biocompatibility testing, chemistry testing, toxicological risk assessment, microbiology testing, large animal studies, and pathology research.
Our center strictly adheres to global regulatory guidelines, as demonstrated by our accreditations from FDA GLP, FDA ASCA, OECD GLP, CNAS/A2LA ISO17025, and CMA. Our facilities are AAALAC-certified and equipped with state-of-the-art operating rooms and laboratories. Our team of professionals, including animal surgeons, veterinary pathologists, and toxicologists, hold dual certifications from both the US and China. This exceptional blend of expertise and accreditation enables us to deliver testing reports that meet global registration requirements, embodying our commitment to “One report, Global submission”.
WuXi Clinical has ability to provide clinical studies and product registration services for medical devices from phase I to IV.
WuXi MedKey has rich experience in operating more than 200 medical device clinical trials and working closely with 1000 Chinese hospital clinical trial centers.



 Large animal experiment
Large animal experiment Pathology Services
Pathology Services Chemical testing
Chemical testing Toxicological risk assessment
Toxicological risk assessment Microbiological test
Microbiological test Biocompatibility
Biocompatibility Biological evaluation
Biological evaluation Product registration
Product registration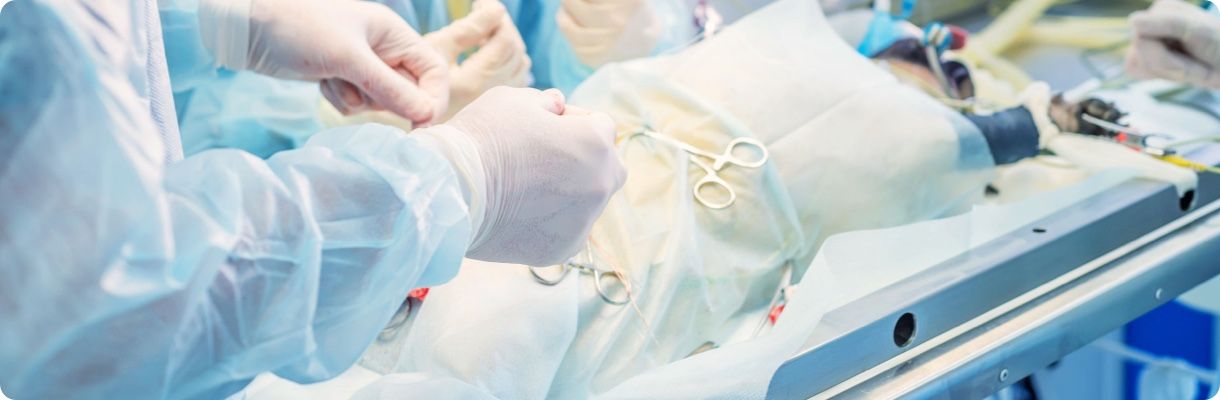
2023-02-16
WuXi AppTec Named “Industry Mover” in S&P Global Sustainability Yearbook 2023
2023-02-12
Global Preclinical CRO Market 2023 by Company, Regions, Type and Application, Forecast to 2029
2023-01-06
Focusing on heart rhythm and heart failure treatment, Wushuang Medical completed hundreds of millions of RMB in Series B+ financing
