Focusing on heart rhythm and heart failure treatment, Wushuang Medical completed hundreds of millions of RMB in Series B+ financing
On January 6, Suzhou Wushuang Medical Equipment Co., Ltd. (hereinafter referred to as "Wushuang Medical") announced the completion of a B+ round of financing of hundreds of millions of yuan. This round of financing was invested by institutions such as Hetang Venture Capital and Kangyu Capital, and the existing shareholder Qiming Venture Capital continued to support it. Founded in 2017, Wushuang Medical is a high-tech medical device company that independently develops a heart rhythm and heart failure treatment technology platform. The company's chief executive officer (CEO) is Dr. Lichuan Hira. According to public information, the success rate of out-of-hospital rescue of sudden cardiac death is extremely low, even less than 1%. Implantable cardioverter-defibrillator (ICD) can effectively prevent sudden cardiac death. It is implanted in the body and is called "the ambulance that protects the heart 24 hours a day". However, ICD product research and development covers high-threshold technologies such as electrophysiology, biomedical engineering, biomedical materials, precision machining, integrated circuits, wireless communications, high-energy battery capacitors, and electrocardiographic algorithms, making comprehensive research and development difficult. According to the press release of Wushuang Medical, the company started with ICD, the technical commanding point in the field of heart rhythm management, and built a technology platform for heart rhythm and heart failure treatment. Centering on heart health, it has built four aspects including prevention, diagnosis, treatment and monitoring. The product line includes ICD system, new pacing system, implantable and wearable heart monitoring system, digital arrhythmia cloud platform, etc. It is reported that the ICD products independently developed by Wushuang Medical have basic functions such as automatic sensing pacing, 40J energy charge and discharge within 10s, synchronous/asynchronous defibrillation, anti-tachycardia pacing, supraventricular tachycardia identification, DF-4 interface, etc., and The rounded streamlined design and the function of Bluetooth telemetry. For this round of financing, Wushuang Medical expressed its gratitude to the new and old shareholders for their recognition and support of Wushuang Medical. Peerless Medical insists on research and development with great concentration, and has been deeply involved in the field of ICD, the commanding height of cardiac rhythm management (CRM) technology for many years. The product has passed type inspection and animal experiments and has laid a solid foundation. It is now ushering in the final touchstone test with clinical experts, looking forward to the ICD of Peerless Medical The products benefit the public and save as many people from sudden cardiac death as possible. Dai Chen, managing director of Hetang Venture Capital, said that cardiovascular is the most important field of medical device layout. Wushuang Medical's ICD and other products have extremely high technical barriers, and subsequent product iterations and chip research and development have also injected high technological attributes into the company. Hetang Venture Capital is firmly optimistic about the Peerless Medical team, through continuous technological innovation, data application innovation and business model innovation, to create an innovative heart rhythm and heart failure treatment technology platform. Wang Hongwei, founding partner of Kangyu Capital, said that there are more than 10 million patients with arrhythmia and heart failure in China. With the formation of expert consensus in specific diagnosis and treatment fields of clinical CRM, China's CRM will have opportunities for high growth in potential product areas. Wushuang Medical is deeply involved in the field of CRM. Based on ICD research and development technology, it realizes the layout of cutting-edge new pacemakers and other products, provides integrated solutions for heart failure and CRM management and treatment, and actively deploys external defibrillation, heart failure monitoring products and digital Medical care will form an overall business closed loop in the future. Kangyu Capital will be optimistic about the empowering value of Wushuang Medical's team, products and business model in data-driven individual heart-managed medical care for Chinese heart patients in the long run. Zhang Ao, executive director of Qiming Venture Capital, said that there are unsatisfied medical needs in China's cardiac rhythm management market segment. Since 2020, Qiming Venture Capital has invested in Wushuang Medical for three consecutive times. It continues to be optimistic about the team's innovation capabilities and company layout. It also believes that the clinical trials of Wushuang Medical in 2023 will be successfully completed. It is hoped that Wushuang Medical will continue to launch innovative products in the near future. Global innovative products such as pacing systems benefit patients all over the world.
On January 6, Suzhou Wushuang Medical Equipment Co., Ltd. (hereinafter referred to as "Wushuang Medical") announced the completion of a B+ round of financing of hundreds of millions of yuan. This round of financing was invested by institutions such as Hetang Venture Capital and Kangyu Capital, and the existing shareholder Qiming Venture Capital continued to support it. Founded in 2017, Wushuang Medical is a high-tech medical device company that independently develops a heart rhythm and heart failure treatment technology platform. The company's chief executive officer (CEO) is Dr. Lichuan Hira. According to public information, the success rate of out-of-hospital rescue of sudden cardiac death is extremely low, even less than 1%. Implantable cardioverter-defibrillator (ICD) can effectively prevent sudden cardiac death. It is implanted in the body and is called "the ambulance that protects the heart 24 hours a day". However, ICD product research and development covers high-threshold technologies such as electrophysiology, biomedical engineering, biomedical materials, precision machining, integrated circuits, wireless communications, high-energy battery capacitors, and electrocardiographic algorithms, making comprehensive research and development difficult. According to the press release of Wushuang Medical, the company started with ICD, the technical commanding point in the field of heart rhythm management, and built a technology platform for heart rhythm and heart failure treatment. Centering on heart health, it has built four aspects including prevention, diagnosis, treatment and monitoring. The product line includes ICD system, new pacing system, implantable and wearable heart monitoring system, digital arrhythmia cloud platform, etc. It is reported that the ICD products independently developed by Wushuang Medical have basic functions such as automatic sensing pacing, 40J energy charge and discharge within 10s, synchronous/asynchronous defibrillation, anti-tachycardia pacing, supraventricular tachycardia identification, DF-4 interface, etc., and The rounded streamlined design and the function of Bluetooth telemetry. For this round of financing, Wushuang Medical expressed its gratitude to the new and old shareholders for their recognition and support of Wushuang Medical. Peerless Medical insists on research and development with great concentration, and has been deeply involved in the field of ICD, the commanding height of cardiac rhythm management (CRM) technology for many years. The product has passed type inspection and animal experiments and has laid a solid foundation. It is now ushering in the final touchstone test with clinical experts, looking forward to the ICD of Peerless Medical The products benefit the public and save as many people from sudden cardiac death as possible. Dai Chen, managing director of Hetang Venture Capital, said that cardiovascular is the most important field of medical device layout. Wushuang Medical's ICD and other products have extremely high technical barriers, and subsequent product iterations and chip research and development have also injected high technological attributes into the company. Hetang Venture Capital is firmly optimistic about the Peerless Medical team, through continuous technological innovation, data application innovation and business model innovation, to create an innovative heart rhythm and heart failure treatment technology platform. Wang Hongwei, founding partner of Kangyu Capital, said that there are more than 10 million patients with arrhythmia and heart failure in China. With the formation of expert consensus in specific diagnosis and treatment fields of clinical CRM, China's CRM will have opportunities for high growth in potential product areas. Wushuang Medical is deeply involved in the field of CRM. Based on ICD research and development technology, it realizes the layout of cutting-edge new pacemakers and other products, provides integrated solutions for heart failure and CRM management and treatment, and actively deploys external defibrillation, heart failure monitoring products and digital Medical care will form an overall business closed loop in the future. Kangyu Capital will be optimistic about the empowering value of Wushuang Medical's team, products and business model in data-driven individual heart-managed medical care for Chinese heart patients in the long run. Zhang Ao, executive director of Qiming Venture Capital, said that there are unsatisfied medical needs in China's cardiac rhythm management market segment. Since 2020, Qiming Venture Capital has invested in Wushuang Medical for three consecutive times. It continues to be optimistic about the team's innovation capabilities and company layout. It also believes that the clinical trials of Wushuang Medical in 2023 will be successfully completed. It is hoped that Wushuang Medical will continue to launch innovative products in the near future. Global innovative products such as pacing systems benefit patients all over the world.

Sino Medical's Non-Compliant Balloon Dilation Catheter Approved for Marketing in the United States
On December 23, Sino Medical Science and Technology Co., Ltd. ("Sino Medical", 688108.SH), its NC ROCKSTAR non-compliant balloon dilation Obtained FDA certification (510(k)). The NC ROCKSTAR non-compliant balloon dilatation catheter that has obtained FDA certification (510(k)) is the first FDA-approved product of Sino Medical, which is suitable for improving myocardial perfusion in patients with atherosclerosis and targeting autologous coronary arteries It is also suitable for post-delivery dilation of balloon-expandable stents (bare metal and drug-eluting stents). The product is designed with a double-layer balloon, which balances the requirements of high burst pressure and low compliance. On the other hand, the shorter shoulder design and the three-layer inner tube design can ensure higher accuracy of balloon expansion, provide better tracking performance and compression resistance, and effectively prevent the guide wire from locking during the operation. Compared with other similar products on the market, it has a smaller passing outer diameter, which can realize full-size kissing with 2.00-4.00mm diameter balloons. This product has been awarded the "Medical Device Registration Certificate of the People's Republic of China" issued by the State Food and Drug Administration on October 28,2022.
On December 23, Sino Medical Science and Technology Co., Ltd. ("Sino Medical", 688108.SH), its NC ROCKSTAR non-compliant balloon dilation Obtained FDA certification (510(k)). The NC ROCKSTAR non-compliant balloon dilatation catheter that has obtained FDA certification (510(k)) is the first FDA-approved product of Sino Medical, which is suitable for improving myocardial perfusion in patients with atherosclerosis and targeting autologous coronary arteries It is also suitable for post-delivery dilation of balloon-expandable stents (bare metal and drug-eluting stents). The product is designed with a double-layer balloon, which balances the requirements of high burst pressure and low compliance. On the other hand, the shorter shoulder design and the three-layer inner tube design can ensure higher accuracy of balloon expansion, provide better tracking performance and compression resistance, and effectively prevent the guide wire from locking during the operation. Compared with other similar products on the market, it has a smaller passing outer diameter, which can realize full-size kissing with 2.00-4.00mm diameter balloons. This product has been awarded the "Medical Device Registration Certificate of the People's Republic of China" issued by the State Food and Drug Administration on October 28,2022.

Bluesail Medical Xintu Microcatheter Approved for Listing in China
Recently, Bluesail Medical Co., Ltd. (“Bluesail Medical”, stock code: 002382) announced that its subsidiary Shandong Jiwei Medical Products Co., Ltd. (referred to as "Jiwei Medical") developed and produced three types of medical device products "microcatheter" approved and registered by the Chinese State Food and Drug Administration. Xintu microcatheter is a dilating microcatheter (piercing catheter) independently developed by Jiwei Medical, which is suitable for coronary artery CTO (chronic total occlusion), severe twisted and bifurcated lesions, severe calcified lesions, etc. In the case of passing through the lesion, it can strengthen the support of the guide wire or be used for exchanging the guide wire. In addition to the functions of ordinary microcatheters, Xintu microcatheters can quickly pass through and expand the twisted microchannels of CTO lesions due to the tapered tip of the helical structure and the elongated hydrophilic coating design, and the ability to open CTO lesions is relatively low. Strong, and can reduce or replace the use of pre-dilated balloons to a certain extent.
Recently, Bluesail Medical Co., Ltd. (“Bluesail Medical”, stock code: 002382) announced that its subsidiary Shandong Jiwei Medical Products Co., Ltd. (referred to as "Jiwei Medical") developed and produced three types of medical device products "microcatheter" approved and registered by the Chinese State Food and Drug Administration. Xintu microcatheter is a dilating microcatheter (piercing catheter) independently developed by Jiwei Medical, which is suitable for coronary artery CTO (chronic total occlusion), severe twisted and bifurcated lesions, severe calcified lesions, etc. In the case of passing through the lesion, it can strengthen the support of the guide wire or be used for exchanging the guide wire. In addition to the functions of ordinary microcatheters, Xintu microcatheters can quickly pass through and expand the twisted microchannels of CTO lesions due to the tapered tip of the helical structure and the elongated hydrophilic coating design, and the ability to open CTO lesions is relatively low. Strong, and can reduce or replace the use of pre-dilated balloons to a certain extent.
Intravascular Ultrasound Diagnostic Equipment" and "Disposable Intravascular Ultrasound Diagnostic Catheter" were approved for marketing
Recently,“Intravascular Ultrasonic Diagnostic Equipment" produced by Shenzhen Sonoscape and the "Disposable Intravascular Ultrasound Diagnostic Catheter" produced by Shanghai Aisheng Biomedical Technology Co, Ltd. Have been approved by Chinese State Drug Administration. The two products are used together for ultrasonic imaging examination of coronary artery vascular lesions. Intravascular Ultrasound Diagnostic Equipment adopts all-digital signal transmission and single-array element mechanical rotation design. Digital transmission is realized between the catheter controller and the imaging host, which improves the integration and stability of the system while reducing the cost and making the image noise more stable. Small. Disposable intravascular ultrasonic diagnostic catheter, on the PMN-PT high-frequency single crystal composite transducer, the process design and method of preparing a glue-free layer with a thickness ≤ 15 μm by evaporation method achieves high-performance high-frequency, this method of the production of the transducer improves the image resolution while optimizing the image quality. This supporting product uses high-frequency, wide-bandwidth, and high-sensitivity ultrasonic imaging, which can realize coronary artery scanning imaging and blood vessel diameter measurement, helping Doctors judge the severity and nature of lesions, which is helpful to improve the understanding of coronary artery lesions and guide interventional treatment.
Recently,“Intravascular Ultrasonic Diagnostic Equipment" produced by Shenzhen Sonoscape and the "Disposable Intravascular Ultrasound Diagnostic Catheter" produced by Shanghai Aisheng Biomedical Technology Co, Ltd. Have been approved by Chinese State Drug Administration. The two products are used together for ultrasonic imaging examination of coronary artery vascular lesions. Intravascular Ultrasound Diagnostic Equipment adopts all-digital signal transmission and single-array element mechanical rotation design. Digital transmission is realized between the catheter controller and the imaging host, which improves the integration and stability of the system while reducing the cost and making the image noise more stable. Small. Disposable intravascular ultrasonic diagnostic catheter, on the PMN-PT high-frequency single crystal composite transducer, the process design and method of preparing a glue-free layer with a thickness ≤ 15 μm by evaporation method achieves high-performance high-frequency, this method of the production of the transducer improves the image resolution while optimizing the image quality. This supporting product uses high-frequency, wide-bandwidth, and high-sensitivity ultrasonic imaging, which can realize coronary artery scanning imaging and blood vessel diameter measurement, helping Doctors judge the severity and nature of lesions, which is helpful to improve the understanding of coronary artery lesions and guide interventional treatment.
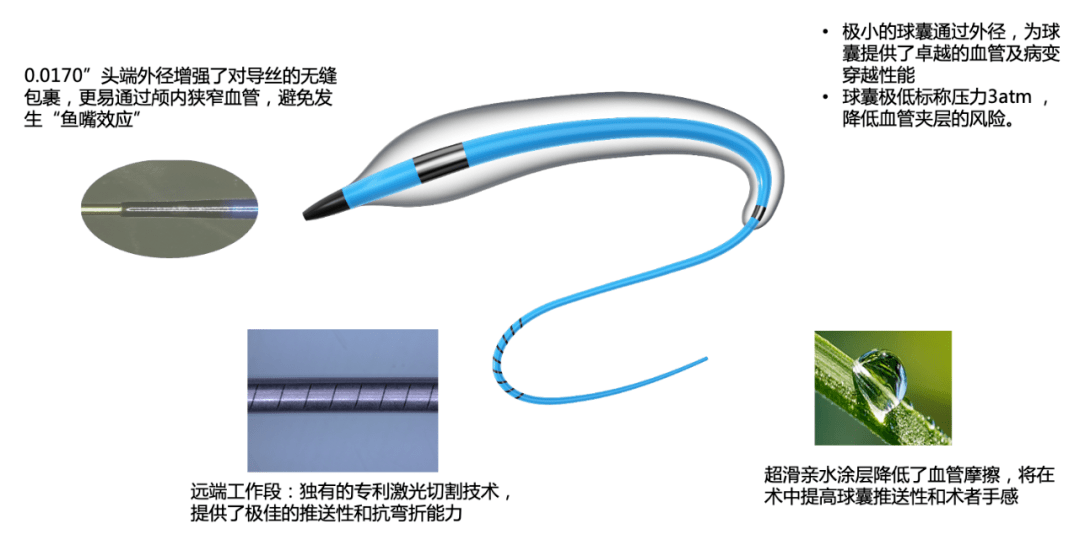
Adtech Medical's NEO-Skater was approved for listing in China
On December 15, 2022, Adtech Medical Technology Holdings Co., Ltd. (6669.HK) announced that its intracranial PTA balloon NEO-Skater for atherosclerotic intracranial blood perfusion has been registered and approved by the Chinese State Food and Drug Administration. NEO-Skater has improved the lubricious coating of the catheter platform and balloon to ensure smooth passage in the tortuous and narrow vascular environment.
On December 15, 2022, Adtech Medical Technology Holdings Co., Ltd. (6669.HK) announced that its intracranial PTA balloon NEO-Skater for atherosclerotic intracranial blood perfusion has been registered and approved by the Chinese State Food and Drug Administration. NEO-Skater has improved the lubricious coating of the catheter platform and balloon to ensure smooth passage in the tortuous and narrow vascular environment.