 Global certified toxicologist, pathologist, experienced veterinary team
Global certified toxicologist, pathologist, experienced veterinary team  270,000+ biocompatibility studies
270,000+ biocompatibility studies  Comprehensive testing capabilities for both in vivo and in vitro
Comprehensive testing capabilities for both in vivo and in vitro In vivo test
Irritation test:
Intracutaneous reactivity test, Skin irritation test, Vaginal Irritation Test, Rectal Irritation Test, Oral Mucosa Irritation Test Test system: Rabbit, Syrian hamsters
Pyrogen test:
Chinese Pharmacopoeia, US Pharmacopoeia, Test system: Rabbit
Implantation test:
Subcutaneous implantation, Intramuscular implantation, Bone implantation, Test system: Rabbit, Rat
Systemic toxicity test:
Acute, Sub-acute, Sub-chronic, Chronic test system: Rat, Rabbit
Sensitization test:
Guinea pig maximization sensitization test (GPMT)., Closed-patch test (Buehler test) Test system: Guinea-pig
Thrombosis test:
Test system: Beagle





In vitro test
Cytotoxicity

MEM elution method

MTT method

Agarose overlay method

Direct contact

Neutral Red Method
Genotoxicity

Chromosomal aberrations

Ames Test

Mouse lymphoma test

Micronucleus test
Haemocompatibility

Complement activation

Haemolysis

Partial thromboplastin time measurement

Leukocyte and platelet counts
In vitro Irritation test
Immunotoxicity test
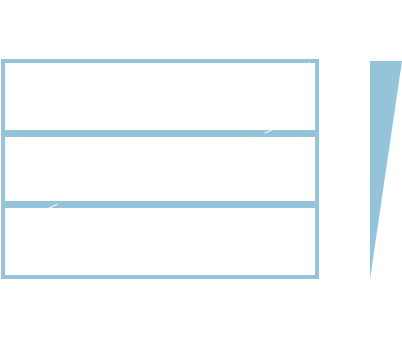
| Stain and Biomarker | Cell type or cellular component evaluated |
|---|---|
| Hematoxylin and eosin (H&E) | All CNS and lymph node tissue |
| Fluoro-jade | Degenerating neurons |
| Autofluorescence | Neurodegeneration |
| Anti-glial fibrillary acidic protein (GFAP) antibody | GFAP (astrocyte biomarker) |
| Anti-iba-1 antibody | Ionized calcium binding adaptor molecule 1 (microglia-specific) |
| Luxol fast blue | Myelin |
Brain implantation test



Virus inactivation validation

Guidelines for technical Review of registration of medical devices of animal origin

Medical Devices Containing Materials Derived from Animal Sources Guidance

Guidelines for review of validation techniques for virus inactivation of allogeneic implantable medical devices

Technical methods and guidelines for verification of virus removal/inactivation from blood products

VIRAL SAFETY EVALUATION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMAL ORIGIN